FDA bans BPA in baby bottles, cups
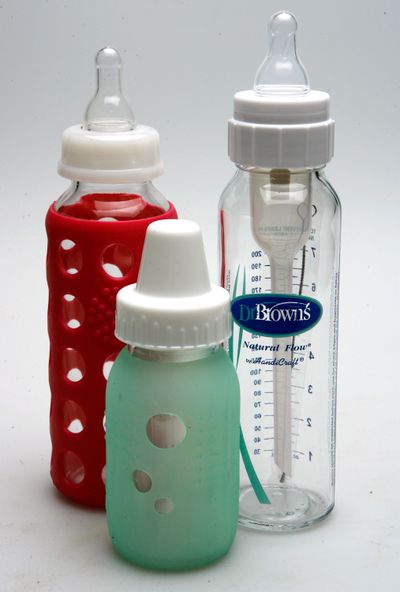
MILWAUKEE — The Food and Drug Administration said Tuesday it is banning the use of BPA in baby bottles and sippy cups, a move critics said did not go far enough and was meaningless because most bottle manufacturers had already phased out the use of the chemical.
The announcement was a response to a request from the chemical industry, which hopes the new ruling will calm public fears about bisphenol-A, a chemical used in many common products, including food containers.
U.S. Rep. Edward Markey, D-Mass., on Tuesday filed a petition to broaden the ban to infant formula cans, and the FDA has opened a 60-day public comment period on the matter.
Meanwhile, health and environmental groups argued the ban should cover all food products, such as the linings of soup cans.
BPA, which is also ubiquitous in cash register receipts and plastic products, has been found to play a role in behavioral problems, diabetes and cancers. An estrogen-like chemical, BPA disrupts the body’s normal hormonal function, and traces of it can be found in the urine of more than 90 percent of Americans.
The FDA has argued that BPA’s harmful effects in rodents don’t apply to humans, and is in the process of conducting its own studies on BPA and human health.
Canada and 11 U.S. states already prohibit BPA in baby products.
The Milwaukee Journal Sentinel’s “Chemical Fallout” investigation found that BPA leached from containers when heated, including those marked “BPA-Free.” The newspaper also revealed that government regulators relied on chemical industry lobbyists to examine BPA’s risks.
Tuesday’s action comes 3 1/2 months after the FDA refused to ban BPA from all food and drink containers, citing inconclusive scientific evidence. That March 30 decision, however, did not endorse BPA as safe.
The tone of the new ruling is much more explicit. “The agency continues to support the safety of BPA for use in products that hold food,” FDA spokesman Curtis Allen said Tuesday. He added that consumers can be confident that baby bottles and sippy cups would be manufactured without BPA.
The Natural Resources Defense Council, which has for years urged the FDA to ban BPA as a food additive, called this move a “half-hearted action” and “inadequate.”
“To truly protect the public, FDA needs to ban BPA from all food packaging,” said Sarah Janssen, senior scientist with the Natural Resources Defense Council, or NRDC.
After the FDA failed to respond to the NRDC’s original 2008 petition to ban BPA in food packaging, the group sued and forced the agency to make a determination, which it did in March.
The FDA’s latest ruling was in response to a petition filed in October by the American Chemistry Council, the U.S. chemical industry’s trade association. The group contends that the plastic-hardening chemical is safe in food and drink packaging.
Daniel Weber, senior scientist at the University of Wisconsin-Milwaukee Children’s Environmental Health Science Center, has tempered enthusiasm for the FDA announcement. His own research has found that BPA affects zebrafish similarly to mercury, causing learning and memory problems.
“We need to understand the effects of chemicals before they are on market,” said Weber, citing thalidomide as an example. “The pharmaceutical industry does rigorous testing, and we need the same kind of thing with chemicals.”
©2012 Milwaukee Journal Sentinel
Visit the Milwaukee Journal Sentinel at www.jsonline.com
Distributed by MCT Information Services